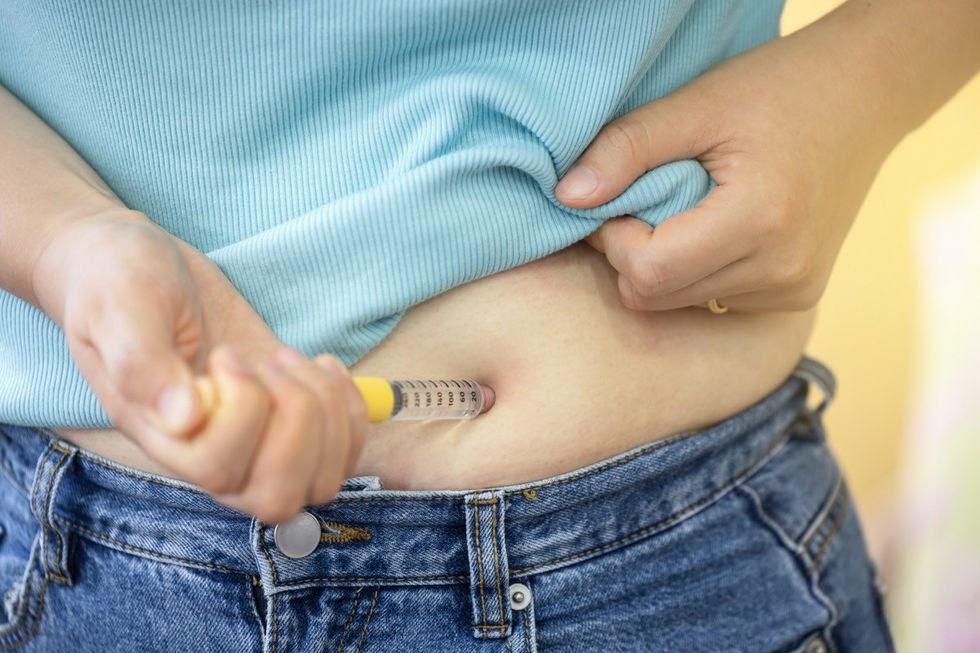
Novo Nordisk, the pharmaceutical company behind the world’s most popular weight-loss injections, is expanding its global market. Wegovy is already available in five other European countries, including Norway, Spain, Switzerland, Italy, and Iceland. However, increasing access to Wegovy elsewhere in Europe has been slow going—and for the French, it’s a little more complicated than just getting a prescription slip.
According to a press release, the French National Agency for the Safety of Medicines and Health Products (ANSM) is “taking measures” to make Wegovy safely accessible to its citizens. However, there are a few caveats.
RELATED: Ozempic Patients Are Switching to These Less Expensive Weight-Loss Drugs.
To start, only endocrinology, diabetology, and nutrition specialists may prescribe the obesity drug. The release explicitly states that general practitioners can only renew GLP-1 medications, not write the “initial prescription.” Additionally, Wegovy will only be available for patients under 65 with a body mass index (BMI) greater than or equal to 35 and will not be paid for by France’s national insurance program.
“Following the opinion of the expert committee, we have decided to restrict the conditions for prescribing and dispensing all GLP-1 indicated in the treatment of obesity. We recommend the use of these drugs in accordance with the care pathway of the High Health Authority (HAS),” the release reads.
Europe is seemingly cracking down on weight-loss drugs like Wegovy, but the French agency cited deductive reasoning, explaining it’s to prevent “cases of misuse with GLP-1” medications.
The agency specifically called out “misappropriation for aesthetic purposes” in the release, adding that Wegovy is “not indicated” for “people who are not obese or overweight, who do not have weight-related health problems.”
In other words, Wegovy is not a form of cosmetic treatment, nor should it be viewed as such. After speaking with a Novo Nordisk spokesperson, Euronews reported that Wegovy will cost Parisians roughly €270 to €330 ($295 to $361) monthly out of pocket.
Wegovy is now available in six European countries, including France, Australia, Brazil, Canada, Japan, and the United Arab Emirates, per Euronews. Ironically, the weight-loss drug isn’t covered in Denmark, Novo Nordisk’s home country, due to what a government advisory group called “major uncertainties” and its potential to create a “socially irresponsible” financial burden on the country.
RELATED: Patient Shares Another Ozempic Side Effect: “You Never Know What Will Set It Off.”
In the U.S., there’s been plenty of talk about the cosmetic use of the treatments, specifically regarding celebrities. In a commercial released earlier this year, Eli Lilly—the manufacturer of type 2 diabetes drug Mounjaro and its sister drug for weight loss, Zepbound—actually addressed patients taking the medications without meeting the approval requirements from the U.S. Food and Drug Administration (FDA).
“Some people have been using medicine never meant for them,” a voiceover says. “For the smaller dress or tux. For a big night. For vanity. But that’s not the point.”
“People whose health is affected by obesity are the reason we work on these medications,” the voiceover continues. “It matters who gets them.”
Speaking with CNN, Eli Lilly CEO David Ricks said there are three main reasons why the company is cracking down on who has access to the medications, namely insurance coverage, shortages, and the type of research required to develop these drugs.
It’s worth noting that insurance coverage is available for some of these treatments, but they usually aren’t covered unless they’re prescribed for type 2 diabetes, according to the National Association of Insurance Commissioners (NAIC). Per GoodRx, coverage for the treatments indicated for weight loss, like Wegovy, “varies widely among insurance plans.”
“We’re a long way from being able to supply a billion people with these medications with obesity, let alone the people who may want to lose some weight cosmetically,” Ricks told CNN. “So we need to prioritize, and that’s what this ad’s about, is prioritizing those who need it most.”
The FDA has also spoken out about people taking the drugs, specifically “unapproved” compounded varieties, for aesthetic purposes.
“FDA is aware that some patients and health care professionals may look to unapproved versions of GLP-1 (glucagon-like peptide-1 (GLP-1) receptor agonists) drugs, including semaglutide and tirzepatide, as an option for weight loss,” an Oct. 2 notice reads. “This can be risky for patients, as unapproved versions do not undergo FDA’s review for safety, effectiveness and quality before they are marketed.”
Making things more complicated, drug manufacturers in the U.S. are also dealing with fake or illegal versions of the medications, CNBC recently reported. In Dec. 2023, the FDA issued an alert about seizing “thousands of units of counterfeit” Ozempic that entered the U.S. supply chain, but there haven’t been any updates to that initial warning, a spokesperson told CNBC.
Whether U.S. health officials will take similar actions to their European counterparts remains to be seen.